Abstract
Introduction: The efficacy and safety of bosutinib (BOS) vs imatinib (IMA) in patients with newly diagnosed chronic phase (CP) chronic myeloid leukemia (CML) was assessed in the phase 3 BFORE trial (NCT02130557) after 5 years of follow-up (Brümmendorfet al., J Clin Oncol, 2022). Here we analyze the impact of comorbidities on efficacy and safety, reporting the results by Charlson Comorbidity Index (CCI) score.
Methods: Patients with newly diagnosed CP CML were randomized 1:1 to receive BOS or IMA (starting dose: 400 mg QD). Outcomes were assessed after 5 years (240 weeks) of follow-up (database lock: June 12, 2020) according to the CCI score without the age component (mCCI). Patients were grouped by mCCI scores of 2 (BOS, n=195; IMA, n=196 [2 untreated]) and >2 (BOS, n=73; IMA, n=72 [1 untreated]). Efficacy was assessed in all randomized patients (intent-to-treat population) and safety in patients who received >1 dose of study medication (safety population).
Results: In patients with mCCI 2 and >2, the median treatment duration was 55.1 and 53.4 months with BOS vs 55.0 and 55.1 months with IMA; respective median (range) dose intensity was 396.4 (39−583) and 367.2 (74−573) mg/day with BOS vs 400.0 (240−765) and 400.0 (189−754) mg/day with IMA. Permanent treatment discontinuations in patients with mCCI 2 and >2, respectively, were reported in 36.4% and 50.7% of patients with BOS vs 45.4% and 32.4% of patients with IMA. The most common primary reasons for permanent discontinuation were adverse events (AEs) in the BOS arm (BOS: mCCI 2, 21.0%; mCCI >2, 35.6%; IMA: mCCI 2, 13.9%; mCCI >2, 8.5%) and lack of efficacy (suboptimal response, treatment failure, or disease progression) in the IMA arm (BOS: mCCI 2, 6.7%; mCCI >2, 2.7%; IMA: mCCI 2, 20.6%; mCCI >2, 9.9%). In both treatment arms, the majority of permanent discontinuations due to AEs occurred in year 1 (BOS: mCCI 2, 11.3%; mCCI >2, 20.5%; IMA: mCCI 2, 7.7%; mCCI >2, 5.6%).
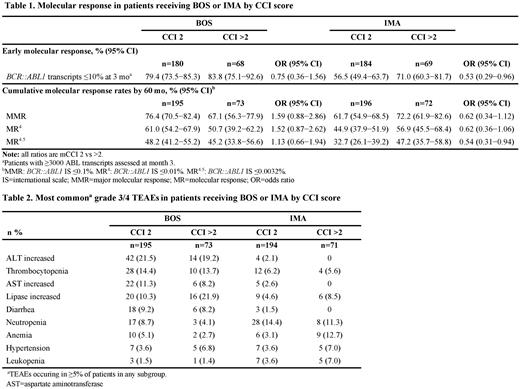
Among patients with mCCI 2 and >2, respectively, the proportion who achieved an early molecular response (BCR::ABL1 ≤10% at 3 months) was 79.4% and 83.8% with BOS vs 56.5% and 71.0% with IMA (Table 1). Cumulative response rates according to mCCI score and treatment arm are shown in the Table 1. There were 14 deaths during the study period in both the BOS- (mCCI 2, n=7; mCCI >2, n=7) and IMA (mCCI 2, n=9; mCCI >2, n=5) arms; 3 and 4 deaths, respectively, were assessed by investigators as CML-related (all in patients with mCCI 2).
Treatment-emergent AEs (TEAEs) occurred in ≥98.5% of patients in each treatment arm and across CCI subgroups. In patient with CCI 2 and >2, respectively, the most common (≥30% in either subgroup) TEAEs with BOS were diarrhea (79.5% and 63.0%), thrombocytopenia (37.4% and 31.5%), nausea (35.9% and 41.1%), increased alanine aminotransferase ([ALT] 34.4% and 31.5%), and fatigue (17.9% and 30.1%). Most common TEAEs with IMA were diarrhea (37.1% and 49.3%), nausea (41.2% and 45.1%), muscle spasms (33.0% and 23.9%), and anemia (17.5% and 36.6%). Among patients with CCI 2 and >2, respectively, grade 3/4 TEAEs were observed in 69.2% and 84.9% with BOS vs 55.2% and 62.0% with IMA. The most frequently reported grade 3/4 TEAEs are shown in Table 2. TEAEs resulting in death in the BOS arm (n=3) were acute cardiac failure, myocardial ischemia, and renal failure (all in patients with CCI >2). TEAEs leading to death in the IMA arm (n=4) were sepsis and CML in patients with CCI 2, and pneumonia and cerebrovascular accident in patients with CCI >2.
Conclusions: After 5 years of follow-up in the BFORE study, a substantial proportion of patients across mCCI scores achieved molecular responses with BOS or IMA. In the BOS arm, there was a trend toward a higher rate (>10% difference) of MR4 in patients with CCI 2 vs CCI >2, with a similar proportion of patients achieving MMR and MR4.5 in both subgroups. Molecular response rates by 60 months were higher with BOS vs IMA in patients with CCI 2 and similar in patients with CCI >2. Safety data were consistent with the known safety profiles of BOS and IMA. Discontinuations due to AEs were higher (>10% difference) in patients with CCI >2 in the bosutinib arm and similar across CCI groups in the imatinib arm. However, in both treatment arms, there were no relevant differences in the frequency of TEAEs across the most commonly occurring AEs between patients with CCI 2 and CCI >2. These results support the use of 400 mg once daily BOS in patients with newly diagnosed CP CML irrespective of baseline comorbidities.
Disclosures
Deininger:Galena Biopharma: Consultancy, Honoraria; Pfizer Inc: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Blueprint Medicines Corporation: Consultancy, Honoraria, Research Funding. Brummendorf:Pfizer Inc: Consultancy, Honoraria, Other: Travel, Accommodation, Expenses, Research Funding; Novartis: Consultancy, Honoraria, Other: Travel, Accommodation, Expenses, Research Funding; Janssen: Consultancy, Other: Travel, Accommodation, Expenses; Merck: Consultancy, Other: Travel, Accommodation, Expenses. Milojkovic:Bristol Meyers Squibb: Consultancy, Honoraria; Pfizer Inc.: Honoraria; Novartis: Honoraria; Incyte: Honoraria, Research Funding. Stenke:Xspray Pharma: Consultancy. Leip:Pfizer: Current Employment, Current equity holder in publicly-traded company. Purcell:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Viqueira:Pfizer Inc: Current Employment, Current equity holder in publicly-traded company. Cortes:Sun Pharma: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Biopath Holdings Inc: Consultancy, Current equity holder in private company; Gilead: Consultancy; Forma Therapeutic: Consultancy; Takeda: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal